Patient safety in medical research is a critical concern that has taken center stage, particularly in the wake of recent challenges in funding and oversight. With the halt in federal research grants, especially those related to medical research funding from the NIH, significant implications arise for the ethical treatment of study participants. The role of institutional review boards (IRB) in ensuring patient safety cannot be overstated, as they navigate complex regulations and safeguard the welfare of those involved in clinical trials. Researchers and institutions must adhere to rigorous standards, and any disruption could dismantle the delicate balance established by extensive research ethics in healthcare. As we explore this pressing issue, it is essential to understand how funding impacts not only the integrity of clinical trial oversight but also the collective trust in the medical research community.
The importance of safeguarding participant welfare in clinical studies has never been more pronounced, especially with discussions surrounding the ethical implications of research activities. Known as the guardians of research ethics, institutional review boards (IRBs) are fundamental in overseeing studies that involve human subjects, ensuring that their rights and safety are prioritized. Moreover, the impact of halted funding on research integrity has raised alarms, with potential detriments to the collaborative spirit that fuels innovation in healthcare. The intersection of compliant research practices and available resources underscores a crucial dialogue about the future of patient protection. With increasing scrutiny on medical research practices and their ethical dimensions, the dialogue surrounding patient safety in research settings continues to evolve.
The Importance of Patient Safety in Medical Research
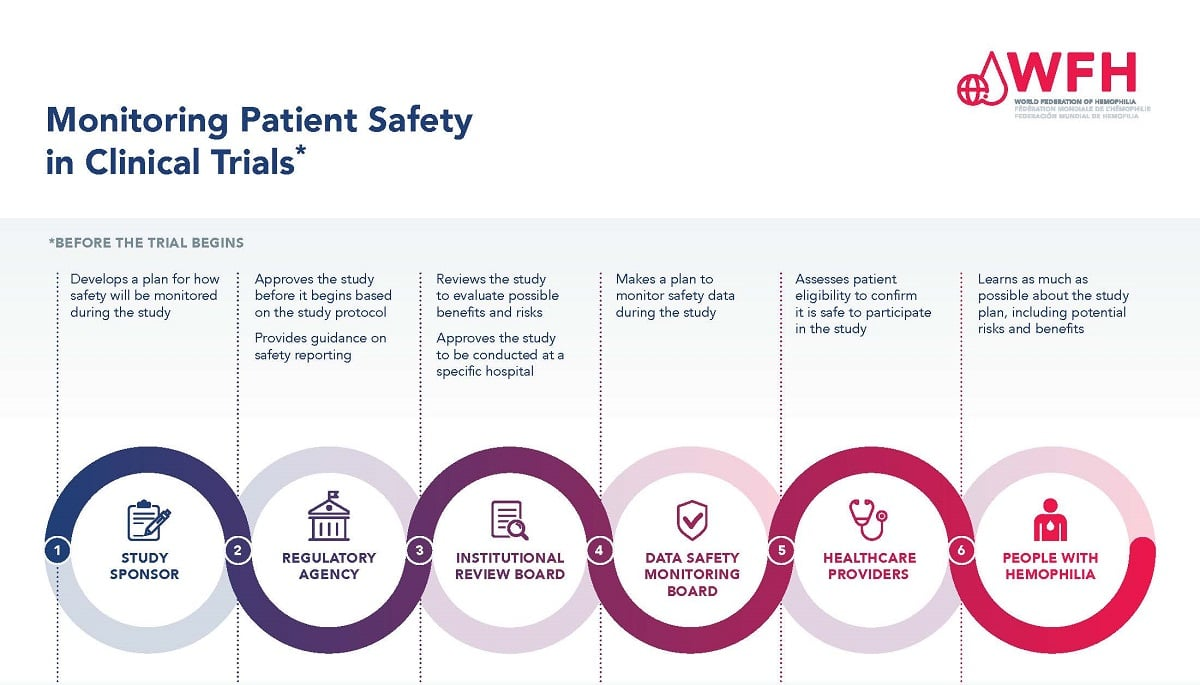
Patient safety is paramount in medical research, as it directly impacts the rights and welfare of individuals who volunteer to participate in clinical trials. The Institutional Review Board (IRB) plays a crucial role in safeguarding these interests by ensuring that all research studies comply with ethical standards and regulations. This oversight includes rigorous scrutiny of research proposals, informed consent processes, and methods to mitigate risks involved in the studies. The involvement of IRBs not only protects patients but also helps build trust in the research process, ensuring that the benefits of findings do not come at the expense of participant safety.
Historically, patient safety has been a significant concern in medical research, stemming from past unethical practices that have led to public distrust. Events such as the Tuskegee Syphilis Study and other unethical research practices have shaped the regulations and oversight frameworks implemented today. Legal requirements and ethical standards like informed consent have emerged directly from these past abuses, highlighting the importance of protecting participants. As research evolves with new treatments and technologies, maintaining a focus on patient safety remains critical to encouraging participation and advancing medical knowledge.
Impact of NIH Funding on Patient Safety Initiatives
NIH funding substantially influences patient safety in medical research. These federal grants provide financial resources essential for conducting studies that follow strict ethical guidelines and research protocols. With proper funding, institutions can allocate necessary resources towards comprehensive IRB reviews, participant education initiatives, and adherence to safety regulations. The loss of these funds threatens to disrupt the established oversight processes, ultimately jeopardizing the safety of participants and the integrity of research outcomes.
Moreover, NIH funding enables collaborative research networks through mechanisms such as the streamlined single IRB (sIRB) model. This model allows for efficient oversight of multisite studies, significantly improving patient safety by ensuring uniform standards across all participating institutions. When funding is cut, the ability to maintain such collaborative efforts is compromised, which could lead to inconsistencies in patient protections nationwide. Ensuring that funding for NIH and similar programs is stable is therefore crucial for sustaining patient safety in the context of contemporary medical research.
The Role of Institutional Review Boards (IRB) in Research
IRBs are integral to the framework of medical research, tasked with the mission to protect human subjects participating in studies. They evaluate research proposals primarily to ensure the safety, rights, and welfare of participants are prioritized. The IRB review process includes the assessment of risks, benefits, and procedures involved in a study, aiming to prevent potential harm. This regulatory body also serves as an educational resource for researchers, equipping them with the knowledge necessary to conduct ethical and responsible studies.
In addition to reviewing research protocols, the IRB also monitors ongoing studies to ensure adherence to ethical standards throughout the research lifecycle. This continual oversight is essential for safeguarding patient safety, as it allows for the early identification and mitigation of risks that may arise during a study. As we reflect on historical cases of unethical research, the importance of a well-functioning IRB system becomes even clearer, serving as a crucial line of defense against exploitation and harm in medical research.
Challenges to Clinical Trial Oversight
Recent federal funding cuts have posed significant challenges to clinical trial oversight, particularly affecting institutions that rely heavily on NIH funding for their research activities. With decreased financial support, many IRBs face the risk of under-resourced operations, which directly impacts their ability to conduct thorough reviews and maintain oversight of ongoing studies. This situation can lead to an environment where patient safety is compromised, as insufficient reviews may overlook critical risk assessments and ethical considerations in research.
Additionally, when clinical trial oversights are hampered by funding constraints, the potential for adverse events increases. Participants may unknowingly be exposed to risks not adequately assessed, leading to physical and emotional harm. The integrity of the research process is also at stake, as studies may experience delays or cancellations that reflect negatively on the involved institutions and diminish public trust. It is imperative to address these challenges with sustainable funding solutions to ensure robust oversight of clinical trials and the safety of research participants.
Research Ethics in Healthcare: A Historical Perspective
The evolution of research ethics in healthcare stems from a history marked by severe violations of human rights during clinical trials. Ethical frameworks like the Belmont Report emerged in the wake of notorious cases such as the Tuskegee Syphilis Experiment, which highlighted the dire need for institutional regulation through IRBs. By instituting protocols that prioritize informed consent and participant welfare, the goal has been to align medical research with ethical standards that uphold human dignity. Today, these historical lessons remain a guiding principle in how healthcare research is conducted.
This ethical evolution is underscored by continuous education and advocacy for human subjects protection within the research community. The establishment of regulatory bodies and ethical review processes exemplifies the commitment to prevent the recurrence of past abuses. Furthermore, incorporating the perspectives of patients and communities in the research design strengthens the ethical foundation upon which modern clinical studies stand. By learning from history, research ethics in healthcare are now more focused on achieving a balance between scientific advancement and maintaining the highest standards of patient safety.
The Effects of Funding Cuts on Patient Engagement
Funding cuts to medical research have broader implications for patient engagement in clinical trials. When federal grants are halted or reduced, opportunities for community involvement in research can diminish, as many patient advocacy and engagement initiatives rely on these funds. A lack of resources directs focus away from efforts to educate and inform potential participants about available studies, ultimately leading to reduced participation rates. This decline not only affects trial outcomes but also limits the diversity of research cohorts, which is critical for ensuring the generalizability of findings.
Furthermore, diminished patient engagement due to funding cuts can exacerbate public mistrust in research studies. When individuals feel disconnected from the research process or are unaware of their rights and the safety measures in place, their willingness to participate may decrease. Such disengagement undermines the progress of research efforts and ultimately affects healthcare advancements. Strengthening funding for patient engagement initiatives is vital for fostering a collaborative and transparent research environment that prioritizes participant welfare.
Innovations in Research Oversight Technologies
As medical research continues to evolve, so do the technologies employed to enhance research oversight and patient safety. Innovations such as electronic data capture systems, remote monitoring tools, and artificial intelligence-driven analytics are becoming integral to how clinical trials are conducted. These technologies streamline data collection, enhance communication between researchers and participants, and ensure real-time monitoring of patient safety. By leveraging these advancements, IRBs can conduct more efficient and effective reviews, leading to better oversight of research protocols.
However, while technological innovations present opportunities, they also pose challenges related to data privacy and participant consent. As research institutions adopt new technologies, it is crucial to establish robust ethical frameworks that protect patient information and ensure informed consent processes remain transparent. Continuous dialogue between technological advancements and ethical considerations is critical to maintaining trust in research practices. As we move forward, balancing innovation with a commitment to patient safety will be essential for the credibility of medical research.
The Future of Patient Safety in Medical Research
The future of patient safety in medical research hinges on robust funding, innovative practices, and unwavering ethical standards. As medical research funding experiences fluctuations, it is crucial that stakeholders advocate for sustainable investments that prioritize patient safety. This includes not only NIH funding but also exploring alternative funding sources that can support ethical oversight and the complex needs of modern research initiatives. Ensuring that patient safety remains a top priority will require concerted efforts from researchers, institutions, and policymakers alike.
Moreover, the commitment to building a culture of safety within research institutions is paramount. Fostering transparent communication, promoting continuous education on ethical standards, and encouraging involvement from the patient community will play significant roles in shaping the future landscape of medical research. By embracing these principles, we can strive toward a research environment where patient safety is not merely an obligation but an inherent aspect of the scientific process, ultimately benefitting participants and advancing healthcare outcomes.
Ensuring Compliance with Ethical Standards
Compliance with ethical standards in medical research is not just a requirement; it is a crucial component of patient safety and trust in the research process. Institutions must ensure that they adhere to established ethical guidelines, such as those set by IRBs and federal regulations, to protect individuals involved in studies. This involves regular training for research teams on best practices, ethical conduct, and the importance of informed consent. With cutting-edge training, researchers are better equipped to uphold the integrity of their studies while effectively safeguarding participant welfare.
Moreover, institutions must foster an environment where ethical concerns can be addressed promptly and transparently. This includes creating pathways for whistleblowing and encouraging researchers and participants to voice their concerns without fear of retaliation. Compliance with ethical standards also entails rigorous monitoring of ongoing studies to ensure that any adverse events are reported and addressed adequately. By prioritizing compliance and establishing a robust framework for ethical research, we can cultivate a research climate that values patient safety and strengthens public trust.
Frequently Asked Questions
How does NIH funding impact patient safety in medical research?
NIH funding plays a critical role in enhancing patient safety in medical research by requiring studies to undergo thorough review by institutional review boards (IRBs). This oversight ensures that the rights and welfare of participants are protected according to ethical guidelines and federal regulations. Moreover, NIH mandates that collaborative multisite research utilize a single IRB for streamlined oversight, ensuring consistent monitoring and adherence to safety protocols across various sites.
What is the role of IRBs in ensuring patient safety during clinical trials?
Institutional review boards (IRBs) are essential for safeguarding patient safety in clinical trials. They rigorously evaluate research proposals, focusing on the study design, risk assessment, informed consent processes, and data monitoring. By doing so, IRBs help to mitigate potential risks and ensure that the rights of study participants are upheld, making them a crucial part of the medical research oversight process.
How do funding cuts affect clinical trial oversight and patient safety?
Funding cuts significantly disrupt clinical trial oversight and patient safety by halting ongoing studies and preventing the addition of new research sites. This can lead to delayed or canceled trials, increasing the risk of harm to participants and decreasing public trust in medical research. Such disruptions hinder the ability of IRBs and research institutions to effectively monitor and ensure the safety of patient participants.
What are the implications of inadequate research ethics in healthcare for patient safety?
Inadequate research ethics in healthcare can severely jeopardize patient safety. When ethical guidelines are not strictly followed, it can lead to improper informed consent, insufficient risk management, and lack of participant support. This undermines the integrity of research studies and potentially exposes participants to undue harm, highlighting the critical need for robust ethical oversight provided by IRBs and funded institutions.
What historical events highlight the importance of patient safety in medical research?
Historical events, such as the Tuskegee syphilis study and unethical experiments during World War II, underscore the dire need for rigorous patient safety protocols in medical research. These incidents led to the establishment of ethical standards and regulatory protections to prevent exploitation and ensure informed consent, shaping the landscape of research ethics that prioritizes patient safety and rights in contemporary clinical trials.
| Key Point | Details |
|---|---|
| Funding Cuts | The Trump administration’s freeze of over $2 billion in federal research grants has disrupted patient safety efforts. |
| IRB Importance | Institutional Review Boards (IRBs) ensure research compliance with safety regulations and safeguard patient rights. |
| Impact on Research | Cuts hinder ongoing studies, limit new research sites, and may prompt public distrust in clinical trials. |
| Community Involvement | IRBs also provide education and support to maintain ethical oversight in medical research. |
| Historical Context | Past ethical breaches necessitated the formation of IRBs to ensure informed consent and participant safety. |
Summary
Patient safety in medical research is critically impacted by the recent funding cuts to federal research grants. These cuts not only disrupt ongoing studies but also erode the trust necessary for successful collaboration between research institutions and communities. With Institutional Review Boards (IRBs) struggling to maintain safety oversight amid reduced funding, innovation in medical research is at risk. It is paramount that we recognize the importance of sustained support for research initiatives to ensure the well-being of participants and to uphold the integrity of the medical research process.